
November 22, 2024
Biopreservation Benchmarking Results from OHRI’s Biotherapeutics Manufacturing Center
The Challenge
Cell therapy manufacturing processes are evolving rapidly, but cryopreservation hasn’t changed much in the last 50 years. Preserving cell therapy potency from bioreactor to bedside remains a major impediment to commercial viability and patient access.
The Use Cases
CryoStasis' Early Access Partners at pharma and biotechnology companies have been clear about their need for extended hypothermic storage time. This would unlock two critical jobs to be done: 1. Improve facilities utilization – i.e. flexibility to move material from one suite to another and between sites without on-call staff and overtime. 2. Enable preparation of doses centrally so they can be shipped to clinical sites in a ready-to-use state without loss of potency.
The Breakthrough
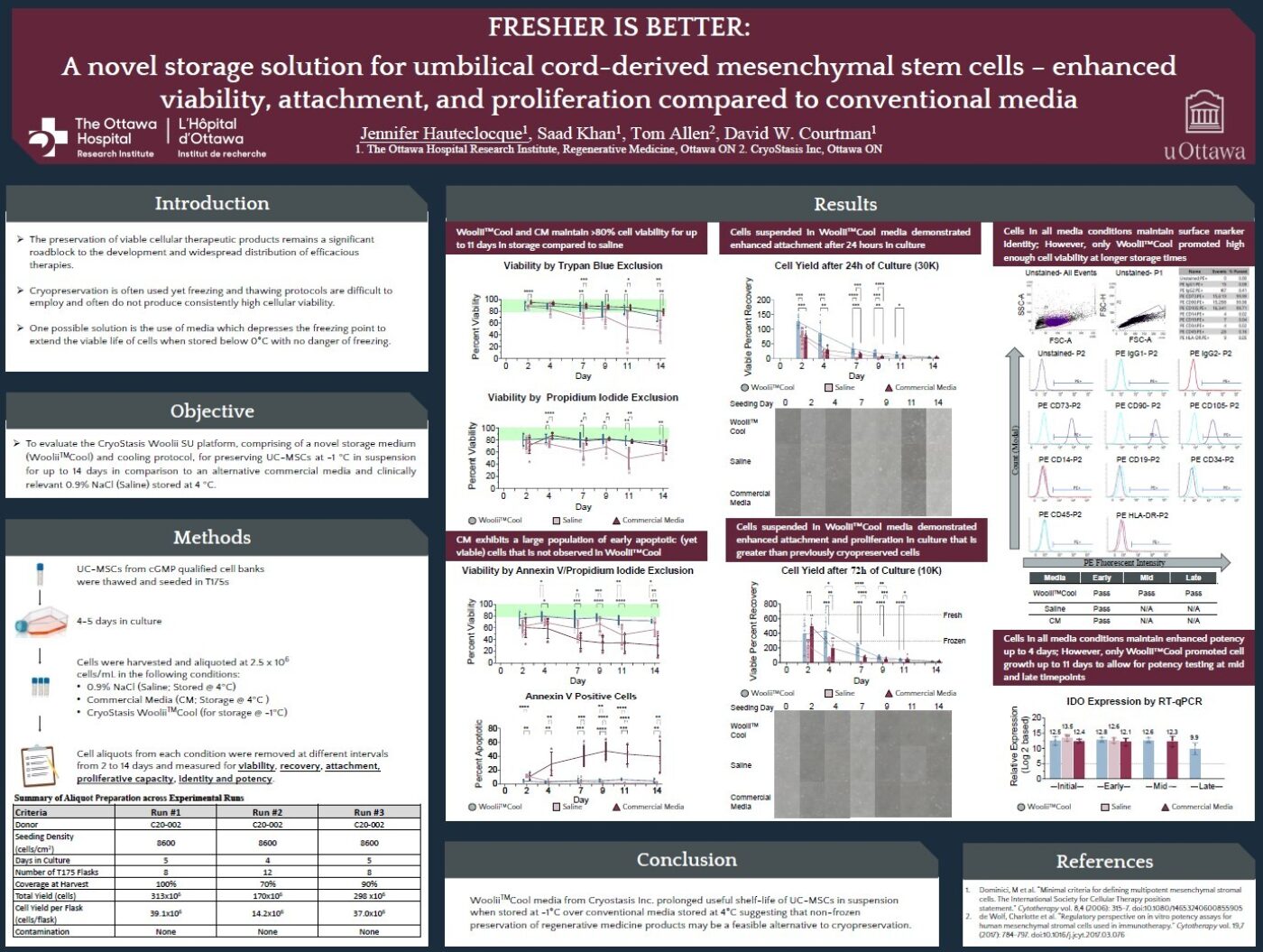
Current commercially available media preserve cells for 2-4 days, but viability and function take a significant hit. Collaborators at OHRI demonstrated that our Woolii™Cool media maintains 80-95% viability of umbilical cord-derived MSCs for up to 11 days when stored subzero unfrozen at -1°C. Furthermore, cells stored in Woolii™Cool showed enhanced attachment and proliferation compared to conventional storage methods - achieving results that even surpassed previously cryopreserved cells.
The Hat-Tip
The Ottawa Hospital Research Institute’s Biotherapeutics Manufacturing Center (BMC) has been pioneering cell therapy manufacturing techniques for over a decade. With scientific freedom to explore what CryoStasis’ solutions can do, our collaborators and research partners are pushing the boundaries of what's possible in regenerative medicine.
For a copy of the poster, send a note to: product@cryostasis.ca
Keywords: MSAT, Process Development, Cryo, Cell Therapy, Biopreservation, CMC, Formulation
We’re on a mission to enable lifesaving research and improve patient outcomes around the world.
Join Us