
Quality program
Quality Policy
CryoStasis commits to manufacturing biological storage and preservation systems that exceed customer expectations and comply with quality, safety, and regulatory standards as well as Good Manufacturing Practices. Our team adheres to a robust quality management system (QMS), which we maintain by continually documenting, understanding, and reviewing quality objectives to ensure we deliver the highest quality solutions and services.
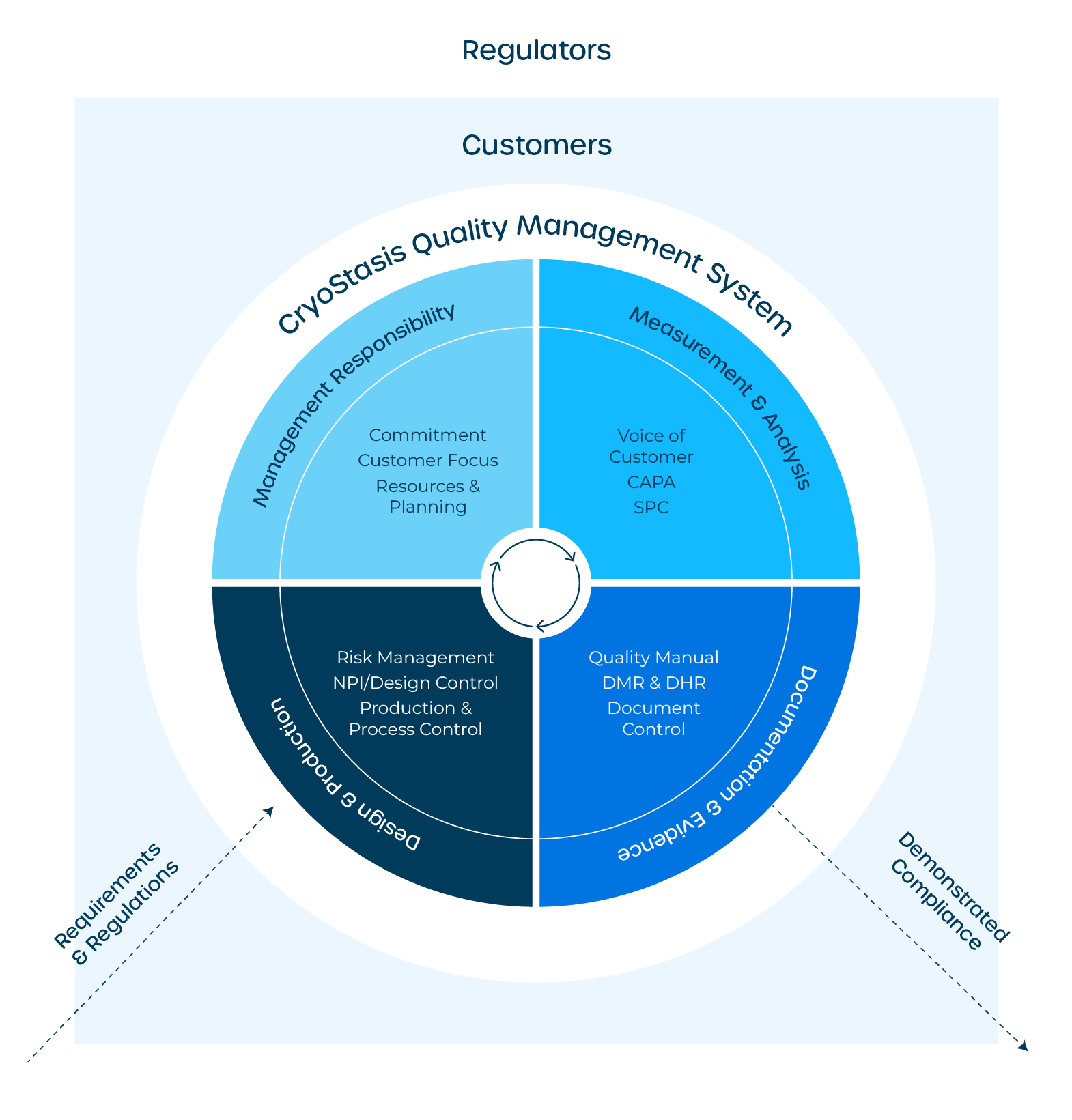
The CryoStasis Quality Management System is built on ISO 13485, ISO 14971, and FDA’s 21 CFR Part 820. The graphic below summarizes our approach to leading with quality and meeting these requirements in a rapidly advancing field.
Media manufacturing is conducted in an ISO 14644-01 [2015] Class 5 vertical laminar flow cabinet within our ISO Class 7 cleanroom.
Other Resources
-
Quality Management System - Management Responsibility
Describes the interdependency of corporate strategy and QMS -
Why Quality Management Systems are critical for Medical Device companies
This article describes the relevance of a QMS for leading medical device companies
We’re on a mission to enable lifesaving research and improve patient outcomes around the world.
Join Us